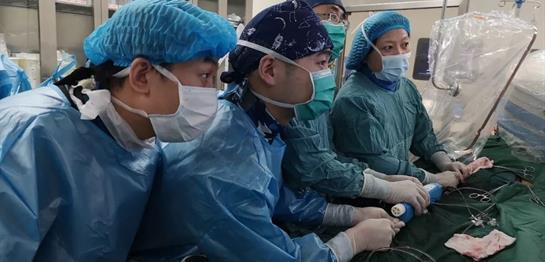
First “post-marketing” implantation in Western China for the TAVI procedure with VitaFlow® was performed at the Department of Cardiology of Xijing Hospital, the surgery was led by cardiology experts Professor Tao Lin and Professor Lifei.
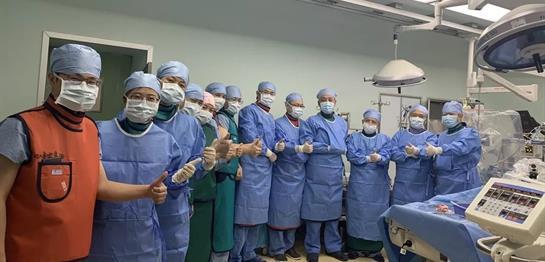
On September 19, 2019, the first “post-marketing” implantation of the VitaFlow® Valve System and Alwide® Balloon in Lingnan Region was successfully performed by the team of Prof. Luo Jianfang at Guangdong General Hospital.
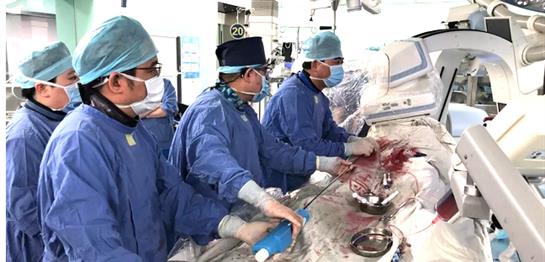
Shanghai, China - on August 28, 2019, the first implantation of the self-developed VitaFlow® Transcatheter Aortic Valve System of MicroPort Cardioflow was performed at Zhongshan Hospital affiliated to Fudan University. Prof. Ge Junbo, Academician of Chinese Academy of Sciences, Director of the Cardiology Department of Zhongshan Hospital, performed the surgery and successfully implanted the VitaFlow® aortic valve into a 70-year-old male patient.

After being approved for the special approval procedure for innovative medical devices, the self-developed VitaFlow® Transcatheter Aortic Valve System (hereinafter referred to as “VitaFlow® Valve System”) of MicroPort CardioFlow successfully obtained the registration certificate from the National Medical Products Administration (NMPA) on July 12, 2019.
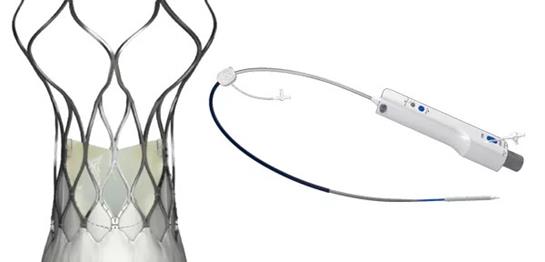
In Shanghai, China on December 12, 2018, the self-developed VitaFlow® II Transcatheter Aortic Valve and Recyclable Delivery System of MicroPort CardioFlow has been admitted into the “Green Path” from National Medical Products Administration (NMPA) for the special approval procedure for innovative medical devices and entered the “Green Path”.
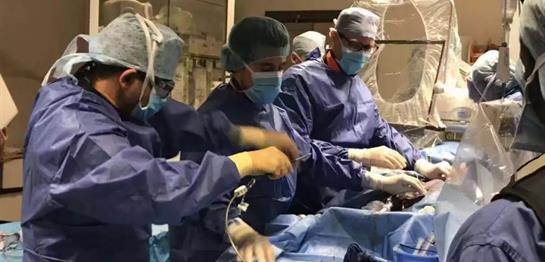
In Galway, Ireland on December 11, 2018, MicroPort CardioFlow announced that the first patient enrollment was completed in Europe for the project of pre-market clinical research on its self-developed VitaFlow™ II Transcatheter Aortic Valve and Recyclable Delivery System (hereinafter referred to as “VitaFlow™ II System”) (“VITALE” project for short).