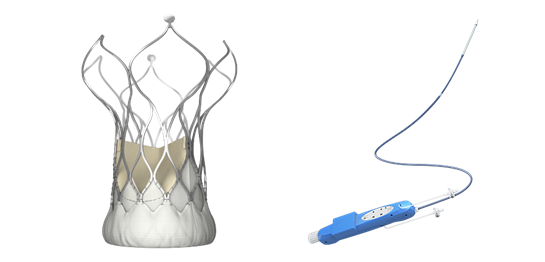
Shanghai, China - VitaFlow® Transcatheter Aortic Valve and Delivery System ("VitaFlow™"), in-house developed by MicroPort CardioFlow, has been admitted into the "Green Path" by the China Food and Drug Administration ("CFDA"), a special fast-track procedure for innovative medical devices to gain CFDA approval.
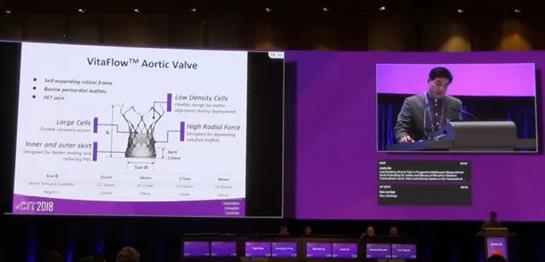
Suzhou, China – From March 22 to March 25, MicroPort Cardioflow, attended CIT 2018 and released the one-year clinical outcome of VitaFlow® Transcatheter Aortic Valve and Delivery System ("VitaFlow®").
On August 22, MicroPort CardioFlow has entered into financing agreements at a consideration of RMB430million, and achieved a valuation of RMB2.1 billion after this round of financing.
MicroPort CardioFlow has recently completed the patient enrollment of the pre-market clinical trial of its self-developed VitaFlow® Transcatheter Aortic Valve and Delivery System ("VitaFlow®"). The clinical trial is a prospective, multi-center trial, aiming at assessing the safety and efficacyof VitaFlow® in the treatment of severe aortic stenosis. The clinical trial was organized by Professor Junbo Ge a renowned cardiologist.