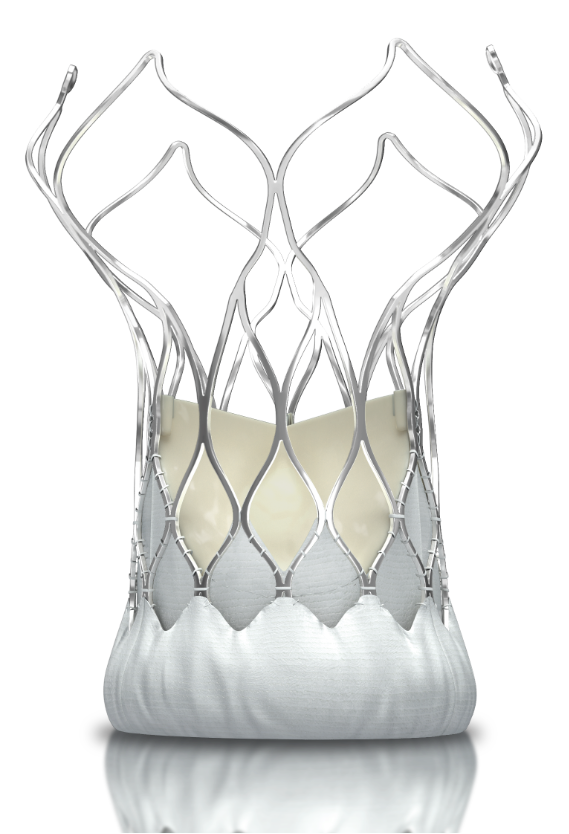
Aortic stenosis is one of the most common and serious valvular diseases. Transcatheter aortic valve implantation (TAVI) as a clinical interventional therapy, provides an ideal alternative treatment for critically ill patients with aortic stenosis who can not tolerate valve replacement through surgical means. With the increasing aging of the population and the expansion of the TAVI indications.