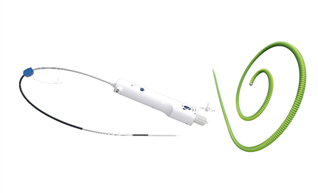
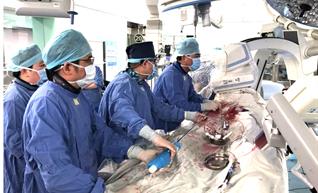
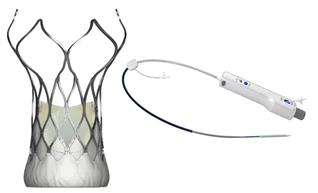
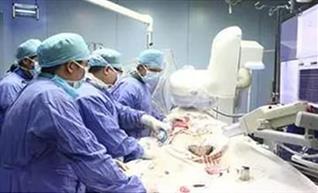
Completed the NMPA submission for VitaFlow® for review
Completed first retrievable VitaFlow Liberty™ implantation for Registration Clinical Trial in China
VitaFlow Liberty™ was admitted into the Green Path for Innovative Medical Device by NMPA
Commenced the clinical trial for VitaFlow Liberty™ in Europe for CE Mark
Invested in ValCare and 4C Medical